
Capillarity explains why a piece of cloth or a paper towel gets wet when you hang its lower edge in water. That causes the meniscus to be pulled upward along the glass surface, and water rises up in the tube. The surface tension of the air film is stronger than the surface tension of the water film. There is also surface tension in the film of water that is contact with the glass of the tube, and also in the film of air that is in contact with the glass of the tube. (This curved surface is called the meniscus.) To understand capillarity, you need to know that there is more to surface tension than just at the water surface. The surface of water in the tube curves upward around its edge. The rise of water in a thin tube is called capillary action, or capillarity. That is because surface tension is making the whole bubble pull inward on itself. Have you ever watched a soap bubble being made by waving a bubble wand? It's stretched out at first, but as soon as it leaves the wand, it becomes a sphere. This shrinkage force is called surface tension. This makes the surface tend to shrink parallel to itself.

It is attracted by molecules below it and beside it, but not from above. It is different for a molecule right at the water surface, however. Inside the liquid, any particular water molecule is acted on by attractive forces from all directions. The water molecules in liquid water attract each other.
OXYGEN CHARGE IN WATER FREE
When the ice melts, the molecules become free to pack together more closely. In the ice structure, the molecules have a relatively open arrangement. When the temperature is high enough, the ice melts, because the thermal vibration of the molecules becomes so strong that the hydrogen bonds are broken. The strength of the vibration increases with temperature. Why does ice melt when the melting temperature is reached? In nature, every atom or molecule undergoes a vibration, or “jiggling,” because it has thermal energy. It is weaker than the bonds between the hydrogen and the oxygen but still strong enough to cause water to freeze into ice. The bond that is formed is called a hydrogen bond. When water molecules bond together in a regular structure to form solid ice, the positive sides of the molecules are attracted to the negative sides of adjacent molecules. In nature, electric charges of the same sign repel, and electric charges of different signs attract. Molecules like this, with one side positive and the other side negative, are called polar molecules. The hydrogen “side” of the water molecule has a slightly positive electric charge. This gives the oxygen “side” of the water molecule a slightly negative electric charge. Electrons have a negative electric charge. The electrons that orbit around the three atoms are more strongly attracted to the oxygen atom than to the hydrogen atoms. The three atoms are not arranged in a straight line instead, they form an angle. The two hydrogen atoms are bonded very strongly to the oxygen atom. Water consists of molecules with the composition H2O (two small atoms of hydrogen and one larger atom of oxygen). More solid substances dissolve in water than in any other liquid. Water is also the best all-around solvent. This tends to even out temperature differences on Earth, from day to night and from summer to winter. The heat capacity of water is more than twice the heat capacity of natural mineral and rock material. The heat capacity of a substance is the amount of heat you need to add to a mass of material to raise its temperature by a given amount. For example, the heat capacity of water is higher than just about any other substance.
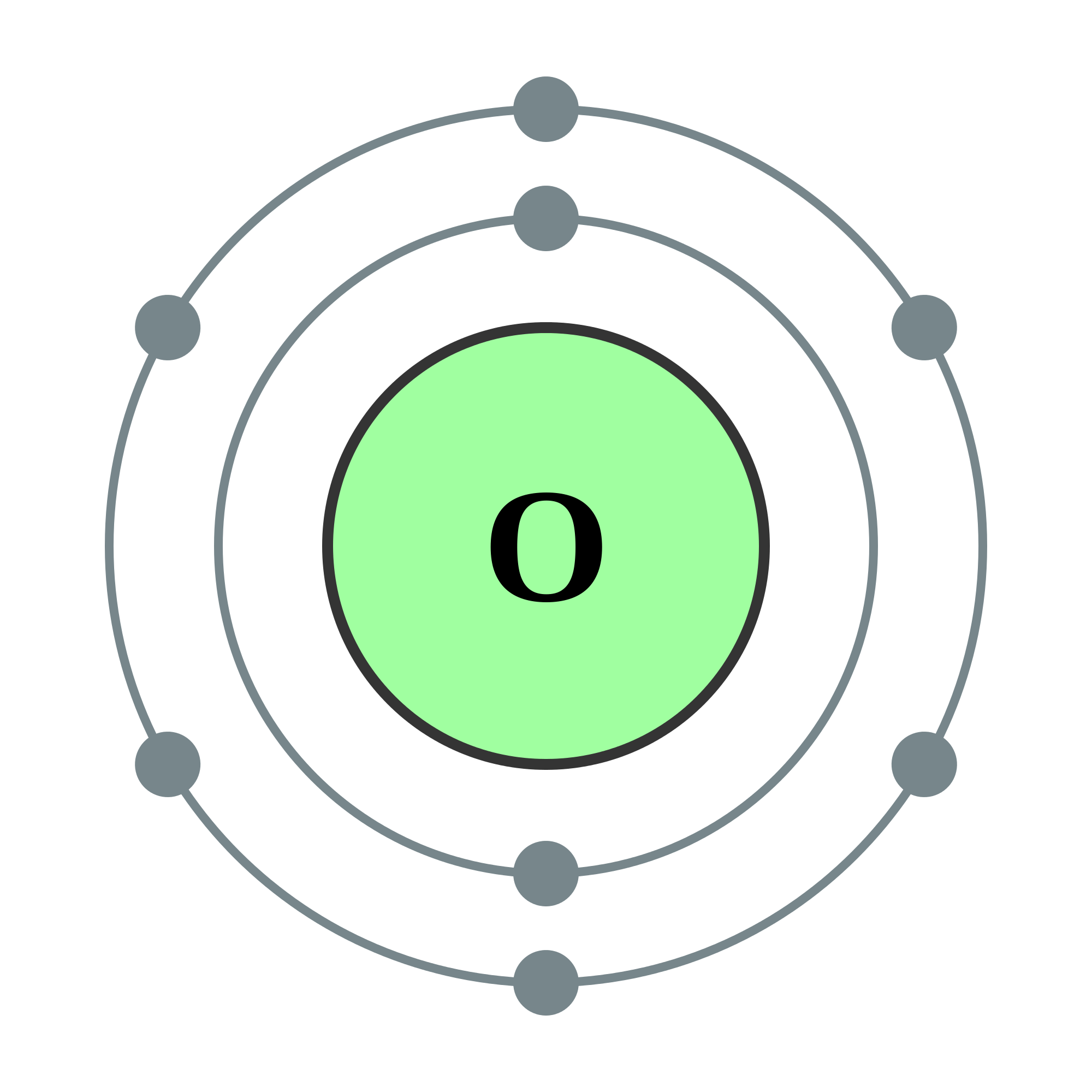
You probably think that's no big deal, but water is almost the only substance in the universe for which the solid floats in the liquid! Water is very unusual in several other ways as well. Its most spectacular property is that ice floats in water. You probably take water for granted because it is so common, but water is a very unusual substance.


 0 kommentar(er)
0 kommentar(er)
